Gingival bleeding in smoking and non-smoking subjects, with or without amalgam fillings, and in correlation with oral hygiene
Lichtenberg, Henrik (Dentist), Torvet 1A, Hilleroed, DK-3400
Ingemarsson-Matzen, Natashia (Dentist, Ph.D., MBA, D. Gem.), Toftegaards Alle 7, Valby, DK-2500
Key words: Smoking – bleeding – amalgam fillings – mercury – gingiva – gum – oral hygiene – gingivitis – periodontitis - periodontal disease – logistic regression – proportional odds model.
Abstract
A clinical study of 1113 patients analysed in a private clinic by regular control for a period of approximately six months. A number of indicative parameters for development of periodontitis was registered in each patient, e.g., bleeding = 0,1,2. Statistical analyses by logistic regression and proportional odds models were used to establish intercausal relations. The models showed that log odds for gingival bleeding above a certain level are a linear function of the explanatory variables. This function was initially assumed to be the same regardless of the level of, e.g. bleeding, and was subsequently expanded for analysis of differences in levels. The explanatory parameters were sex, age, smoking, oral hygiene (three levels) and the presence of amalgam in the teeth. All estimates indicate odds ratios at 95% confidence intervals.
The proportional odds model showed no effect of sex, age or duration of absence of amalgam. Smoking proved to reduce the occurrence of bleeding by an odds ratio of 0.35 (0.20-0.50), impaired and bad oral hygiene increased the occurrence of bleeding by odds ratios of 21.2 (14.2-31.8) and 518 (233-1151). Furthermore, it was
demonstrated that there was no effect of amalgam presence as far as impaired or bad oral hygiene was concerned, but among patients with good oral hygiene, the presence of bleeding was increased by an odds ratio of 3.65 (2.37-5.64).
It was concluded that bad oral hygiene increases the risk of gingival bleeding as does the presence of amalgam fillings (factor 3), whereas smoking is seen to minimise the tendency of bleeding by a factor 3.
Gingival bleeding in smoking and non-smoking subjects, with and without amalgam fillings, and in correlation with oral hygiene
English Summary
A clinical study of 1113 patients in a private clinic is presented. The patients were analysed by a variety of indicative parameters for the development of gingival bleeding.
The outcome analysed was an ordinal response, bleeding = 0,1,2. A proportional odds model was fitted to data. Briefly, the model states that the log odds of bleeding above a certain level are a linear function of the explanatory variables. This function was initially taken to be the same regardless of level, and later expanded with level-interaction. The explanatory variables used in the modelling were age, sex, smoking, oral hygiene (three levels) and presence of amalgam in the teeth.
All estimates are given as odds ratios with 95% confidence intervals.
The proportional odds model showed that there were no effects of sex, age or duration of absence of amalgam. Smoking was found to reduce the presence of bleeding with an odds ratio of 0.35 (0.25 – 0.50), impaired and bad oral hygiene increased the presence of oral bleeding with odds ratios of 21.2 (14.2 – 31.8) and 518 (233 – 1151), respectively. Further, no effect of amalgam presence was found among patients with impaired or bad oral hygiene, but among patients with good
oral hygiene the presence of bleeding was increased by an odds ratio of 3.65 (2.37 – 5.64).
In conclusion, the major risk factor for oral bleeding is oral hygiene, smoking reduces gingival bleeding about three-fold, whereas amalgam presence increases oral bleeding three-fold among patients with good oral hygiene.
Introduction
Gingivitis (gum bleeding) together with caries (holes in the teeth) and periodontitis (inflammation of the periodontal membrane of the teeth in the jaw) are among the most widespread dental diseases. These diseases have dire consequences, not only financially to the individual patient, but often also serious invalidating masticatory effects. (Many people experience tooth loss as a consequence of these diseases to be such an obstacle for their normal behaviour in a masticatory context, and they also experience situations where speaking or singing is so compromised that it could be described as orally invalidating).
The clinical study aimed to establish whether certain influences would increase the possible generation of gingival (gum) bleeding. Is smoking, bad oral hygiene or the presence of amalgam fillings (which has a mercury content of 51%) the decisive factor?
Smoking is often associated with the risk of developing diseases such as cardiovascular diseases, lung cancer and periodontitis (i.e. inflammation of the connective tissue of the teeth, "the disease of the loose teeth") (1 ,2 ,3 ,4 ,5 ,6 ).
These diseases appear to occur more frequently in the western part of the world over the last 20 years. Therefore, worldwide efforts are being made to identify its causes. Smoking is supposed to be one major factor in the development of these diseases.
Studies have shown that, in probing, teeth and the surrounding connective tissue, with recurrent gingival bleeding (i.e. gum bleeding) have a 20-25% higher risk of developing attachment loss within the near future, whereas teeth that do not bleed, with almost 100% certainty will not lose their attachment for a while. In other words, gingival bleeding is a very good tool in diagnosing or predicting whether periodontitis exists or is likely to occur (7 ). In a recent American study, it was claimed that a direct connection had been established between periodontal diseases and smoking (8 ). Based on this and similar studies dentists are requested to take this into consideration in treating or advising patients regarding periodontal diseases.
People with amalgam fillings are exposed to an increased risk of acquiring diseases such as, e.g. cardiovascular diseases, infections and allergies (9 ,10 ,11 ). Tosti et al. (12 ) has documented that allergic stomatitis (inflammation of the oral cavity due to allergic provocation) rarely occurs, but it is nearly always associated with the
occurrence of metallic mercury. Patients with amalgam fillings more frequently also have gingival bleeding (13 ,14 ).
Humans are not the only ones affected by mercury and other heavy metals, other mammals such as, e.g. the porpoise (Phocæna communis) shows sensitivity to the metal, which in this connection supposedly reduces resistence to infectious disease (15 ). Similarly, the UNEP (United Nations Environmental Programme, Division of Technology, Industry and Economics) is generally concerned by the increased occurrence of mercury in our environment (16 ).
Could it be that more frequent gingival bleeding in smokers, with the related risk of developing periodontitis as demonstrated in the American study, is due to the fact that smokers more often have dental amalgam fillings?
This prospective clinical study aims to discuss this, and there is much to indicate that dental amalgam fillings and bad oral hygiene, rather than smoking are the causes that produce gingival bleeding and thus increase the risk of periodontitis.
Material and Method
Four dentists participated in the study of 1113 patients (679 women and 434 men), who attended the clinic for regular control.
A number of parameters were recorded; 1) smoking or non-smoking (273 and 839 persons respectively), 2) age and sex, 3) gingival bleeding, 4) oral hygiene, 5) presence of amalgam fillings or not, 6) years without amalgam.
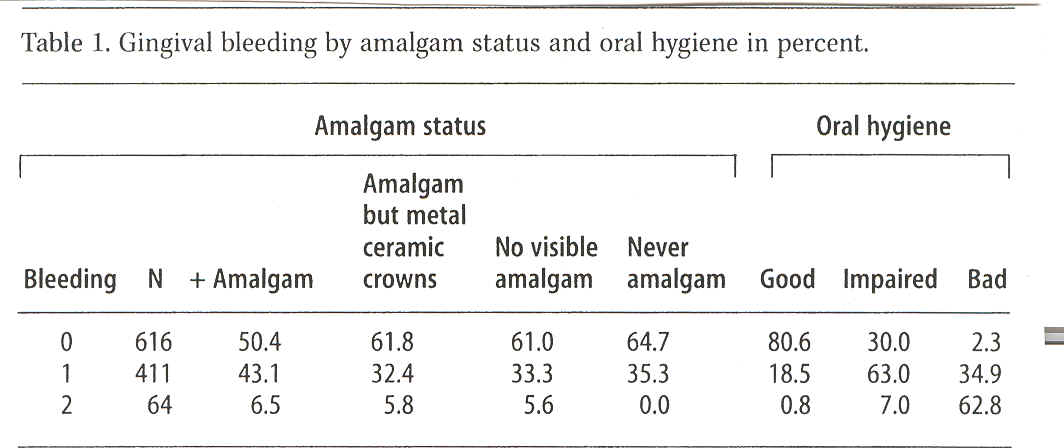
Gingival bleeding was measured and indexed by probing for caries and the subsequent cleaning of teeth with an air scaler (a mechanical tooth cleaning instrument) on each individual patient; the gingiva was categorised by 1) no bleeding (626 persons), 2) moderate bleeding (420 persons) or 3) overall bleeding (67 persons).
Oral hygiene was also assessed in each patient on the basis of the presence of plaque, and oral hygiene was noted to be 1) good (606), 2) impaired (463) or 3) bad (44).
The presence of amalgam fillings was also recorded: 1) did the patient have amalgam fillings (501), 2) did the patient have no amalgam fillings (400), 3) did the patient have no amalgam fillings, but one or more metal crowns on the molars (176), or 4) had the patient never had amalgam fillings (35).
A note was made of how many years the patient had been entirely without amalgam fillings where appropriate.
Statistical Method
For all 1113 patients these variables were entered in the MS Works database system. The statistical analysis included only the patients for whom all parameter groups were present. Therefore, 22 patients had to be excluded from the study. The following analyses and tables were consequently based on 1091 patients (665 women and 426 men). Bleeding by distribution of relevant sub-groups of patients is indicated in per cent in Table 1.

Initially, the outcome of bleeding is dichotomised to be either bleeding (1 or 2) or no bleeding (0), and for this outcome we have the following logistic regression model (17 )
logit(P{Bleeding>0})=logi(p)=ln(p/(1-p))=μ+βk+γa+βh+βr+βA
where the parameters βk, γa, βh, βr and βA refer to the effect of sex: k, age: a, oral hygiene: h, smoking: r and amalgam: A.
In this model gingival bleeding showed no difference between patients as to levels "No", (i.e. no occurrence of amalgam) "- Amalgam visible, but metal ceramic crowns" and "No visible amalgam", and there is no difference between men and women or according to age. Therefore, the model was reduced to a model for estimating odds ratios for gingival bleeding alone, Table 2.

There was no effect of the number of years that the patient had been without amalgam (X2(2)=0.69, p=0.71).
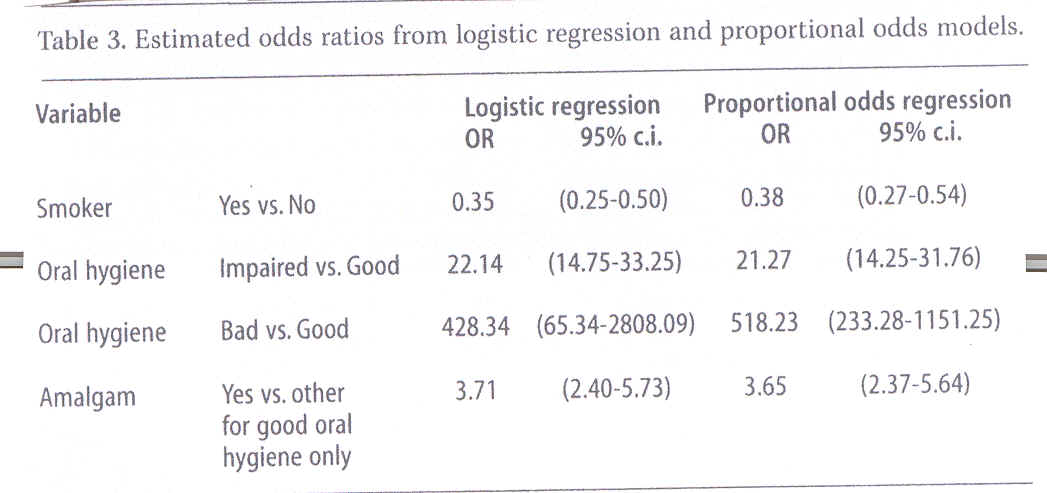
On the other hand, the interaction between amalgam and oral hygiene (X2(2)=20.69, p=0.000) proved significant. A more detailed analysis showed that this interaction could be reduced to show that the effect of amalgam exists only in patients with good oral hygiene, in fact the model describing the data set most adequately was a logistic regression with the effects of smoking, oral hygiene and amalgam presence in patients with good oral hygiene. Estimates for odds ratios from this model are stated in Table 3 to the left.
A more detailed analysis, which takes into account the three-level response, is the so-called proportional odds model - an expansion of the logistic regression model – expressed in an ordinal response:
10
logit(P{Bleeding>i})=logi(pi)=μi+βk+γa+βh+βr+βA, i=0,1
This model illustrates that the effect of co-variables is the same, regardless of bleeding dichotomisation, 0 vs. (1.2) or (0.1) vs. 2.
The data analysis from using this model provided the same conclusions as the logistic regression model shown in Table 2 to the right.
Results
The reviewed population of patients comprised fewer men than women, 39% against 61%, with an age span from 8 to 99 years and about 50 years on average.
Smokers were represented in the group by 25% of the population.
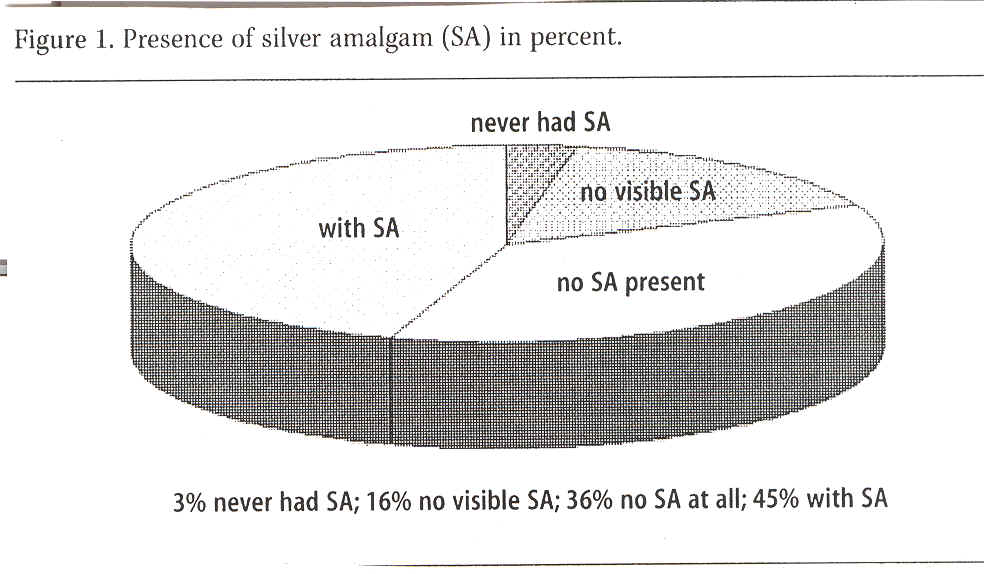
Amalgam presence was recorded in all subjects distributed by 45% of the group with visible amalgam fillings, 16% without visible amalgam fillings, 36% without amalgam fillings (i.e. they had had them previously removed) and 3% of the group, which had never had any amalgam fillings in their teeth. See Figure 1.
Oral hygiene proved to be good in 54%, impaired in 42% and bad in 4% of the group.
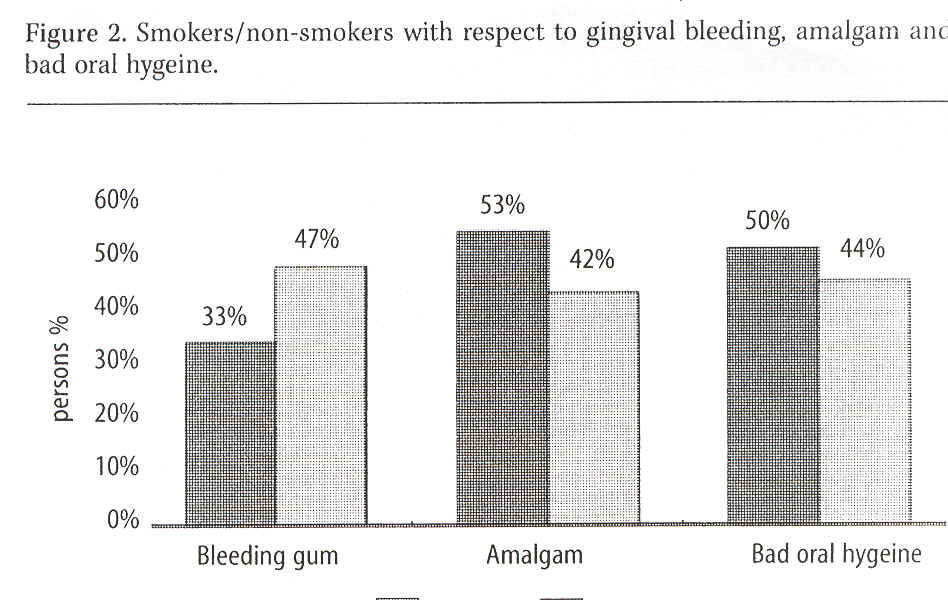
Occurrence of bleeding (i.e. whether gingiva was bleeding when probed or not) was recorded in 56% without bleeding, 38% with some bleeding and 6% with strong gingival bleeding. See Figure 2, which also shows amalgam fillings in smokers and non-smokers. It was observed that 11% more smokers than non-smokers had amalgam fillings.
A comparison of these recordings according to the model system above in the statistical method part established that there was little difference in gingival bleeding levels in the groups "never amalgam", "no visible amalgam" and "no visible amalgam, but metal crowns" and that differences of sex or age did not have any effect on gingival bleeding in this population or the duration of absence of amalgam.
Therefore, the effects of amalgam presence and oral hygiene were the only proved effects, Table 2. It follows that gingival bleeding is three-fold as high in non-smokers as in smokers (OR 0.33 in Table 2).
In terms of oral hygiene "impaired" and "good" were related in the way that "impaired oral hygiene" resulted in gingival bleeding being 11 times as frequent as in "good oral hygiene", but "bad oral hygiene" showed 250 times as frequent an
occurrence of gingival bleeding compared with that in patients with "good oral hygiene".
Also, the effect of amalgam on gingival bleeding was estimated nearly twice as high in patients where amalgam was absent (OR 1.87 in Table 2).
As amalgam and oral hygiene showed important interaction, these parameters were analysed and stated in Table 3. Bad oral hygiene was seen as the predominant risk factor in gingival bleeding; nearly all cases of bleeding in the group of patients with impaired or bad oral hygiene were attributable to this factor. In the group of patients with good oral hygiene 73% (i.e. (3.71-1)/3.71) of gingival bleeding was attributable to the presence of amalgam.
Discussion:
The present study has used avanced statistical analysis to establish the correlation between gingival bleeding on the one hand and smoking, oral hygiene and amalgam presence in the oral cavity on the other.
The relation between gingival bleeding and smoking was negative by an odds ratio of 0.35, whereas gingival bleeding and impaired or bad oral hygiene was positive by
odds ratios of 21.2 and 518, respectively. There is a clear and definite correlation between the degree of bad oral hygiene and the gingival bleeding response.
About 33% of the Danish population are considered smokers, and in our study group this figure was only 25%. This difference may be explained by the health protective image of our dental clinic, which has a reputation for health concerns based on a comprehensive view of humans and the environment, and as smoking can hardly be said to be particularly healthy, groups of smokers may be less inclined to visit our clinic.
For smoking it is a well-known fact that the absence of gingival bleeding may be partly explained by the contraction of peripheral vessels when affected by nicotine. Cyanosis is also known to be a general characteristic found in smokers during regular clinic examination.
Gingival bleeding was lower in smokers in our study despite the fact that smokers more often than non-smokers had amalgam fillings.
An explanation could be found in the fact that nicotine has an inhibitory effect on the transformation of the mercury ion Hg+ to Hg++ (18 ), which is assumed to be more toxic than free mercury.
This seems to indicate that it is not the smoking (nicotine), which is the most important cause of gingivitis (and possible development of periodontitis) but the
amalgam. However, this does not exclude other elements in smoking than nicotine as such from being additive in the development of the periodontal disease (19 ), and we must be very careful in taking this a final conclusion, but merely take notice of this finding in considering the possibility that gingivitis may exist without periodontitis and vice versa.
Our investigation methodology does not provide a clear answer to this question, as we have confined our scope of study to the recording of the existence or not of "gingival bleeding" in various degrees, but have not measured a regular attachment loss clinically or radiologically. In our general clinical opinion there is a clear connection between gingival bleeding on the one hand and the occurrence of periodontitis on the other, with reservations made for causes of gingival cyanosis.
Further research into this aspect is desirable.
In the last two decades periodontitis seems to have become a widespread disease, and may be caused by the general introduction of an amalgam with a large content of copper. In 1984, this type of amalgam was tested by NIOM - Scandinavian Institute of Dental Materials. Researchers established that the "new" amalgam ("High copper amalgam", i.e. amalgam with a high content of copper) emitted 50 times as much copper and mercury to the environment than the previously used type of amalgam (20 ,21 ).
It was noteworthy that amalgam fillings in isolation were seen to be the cause of gingival bleeding in patients with good oral hygiene, with an odds ratio of 3.65, i.e. about 75% of the occurrence of bleeding must be attributable to the presence of amalgam. (Amalgam is a well-known mixture of metals, of which mercury (Hg) forms the major part, succeeded by silver (Ag), copper (Cu), tin (Sn), zinc (Zn) (22 ) and sometimes small amounts of other metals).
There is probably no longer any doubt as to the detrimental effects of mercury generally, and its effects on periodontal tissues as many studies have established (23 ,24 ,25 ,26 ,27 ,28 ,29 ).
As early as in 1973 an investigation showed that the presence of amalgam fillings resulted in chronic inflammation and bleeding in gingival tissues (30 ,31 ,32 ). In other words, amalgam produces chronic gingivitis.
The present study has thus confirmed the hypothesis.
A subsequent study has established that amalgam with a copper content gives off far more mercury than previously used types of amalgam (33 ).
With regard to the effect of oral hygiene on gingival bleeding this study also confirms the significant correlation between oral hygiene and gingival bleeding. This conclusion hardly requires any explanation other than bad oral hygiene is
characterised by large amounts of plaque, which contains myriads of bacteria and that these bacteria are generally more or less pathogenic.
A large number of studies have long established that the removal of plaque from the surfaces of the teeth and gingiva reduces gingivitis as well as any subsequent periodontitis and caries, see the Vipeholm survey 1947-51 (34 ).
Conclusion
The data from this clinical study indicates that gingival bleeding is less frequent in smoking than in non-smoking subjects. Under the assumption that gingival bleeding often precedes the development of periodontitis later, this must imply a reduced risk of periodontitis in smokers.
It has also been demonstrated that smokers have more amalgam fillings than non-smokers.
Not surprisingly, bad oral hygiene often increases gingival bleeding, but it is difficult to expain that the gingiva in smokers shows less bleeding despite the more frequent
occurrence of amalgam fillings, which is known to increase the frequency of gingival bleeding. An explanation could be the stronger effects of tobacco in contracting vessels than the ability of bad oral hygiene to provoke gingival bleeding.
Further clinical studies are desirable as well as investigation into the biological effects of nicotine, or other ingredients, of tobacco on gingival bleeding.
This study has been subsidised by East Denmark Health Science Research Forum, file no. 02013.
Literature
1 . Payne JB, Reinhardt RA, Nummikoski PV, Dunning DG, Patil KD: The association of cigarette smoking with alveolar bone loss in postmenopausal females. J Clin Periodontol 2000; 27: 658-64.
2 . Lukanich JM: Tobacco and public health. Chest 1999; 116 (suppl 6): 486S-489S.
3 . Howard G, Thun MJ: Why is environmental tobacco smoke more strongly associated with coronary heart disease than expected? A review of potential biases and experimental data. Environ Health Perspect 1999; 107 (suppl 6): 853-8.
4 . Zheng T, Holford T, Chen Y, Jiang P, Zhang B, Boyle P: Risk of tongue cancer associated with tobacco smoking and alcohol consumption: a case-control study. Oral Oncol 1997; 33: 82-5.
5 . Albandar JM, Kingman A: Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J Periodontol 1999; 70: 30-43.
6 . Muller HP, Stademann S, Heinecke A: Bleeding on probing in smokers and non-smokers in a steady state plaque environment. Clin Oral Investig 2001: 177-84.
7 . Albandar JM, Brunelle JA, Kingman A: Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol 1999;70: 13-29.
8 . Qandil R, Sandhu HS, Matthews DC: Tobacco smoking and periodontal diseases. J Can Dent Assoc 1997; 63: 187-92, 194-5.
9 . Tomar SL, Asma S: Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol 2000; 71: 743-51.
10 . Siblerud RL: The relationship between mercury from dental amalgam and the cardiovascular system. Sci Total Environ 1990; 99: 23-35.
11 . Trakhtenberg IM. Cardiotoxic effects of mercury. In: Trakhtenberg IM. Chronic effects of mercury on organisms. DHEW Publication No (NIH) 74-473. Dethesda, MD: National Institute of Health, 1974: 199-210.
19
12 . Tosti A, Piraccini BM and Peluso AM: Contact and irritant stomatitis. Semin Cutan Med Surg 1997; 16(4): 314-9.
13 . Siblerud RL: The relationship between mercury from dental amalgam and oral cavity health. Ann Dent 1990; 49 (2): 6-10.
14 . Lichtenberg H: Elimination of symptoms by removal of dental amalgam from mercury poisoned patients, as compared with a control group of impaired patients. J Orthomol Med 1993; 8: 145-8.
15 . Bennett PM, Jepson PD, Law RJ, Jones BR, Kuiken T, Baker JR. Rogan E, Kirkwood JK: Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environ Pollut 2001; 112(1): 33-40.
16 . United Nations Environment Programme, Division of Technology, Industry , and Economics. Global Mercury Assessment 2002; 1-30.
17 . McCullagh P: Regression models for ordinal data (with discussion). J Royal Stat Soc 1980; B42: 109-42.
18 . Freden H, Hellden L, Milleding P: Mercury content in gingival tissues adjacent to amalgam fillings. Odontol Revy 1974; 25: 207-10.
19 . Obeid P, Bercy P: Effects of smoking on periodontal health. Adv Ther 2000; 17: 230-237.
20 . Brune D: Corrosion of amalgams. Scand J Dent Res 1981; 89: 506-14.
21 . Herö H, Brune D, Jörgensen RB, Evje DM: Surface degradation of amalgam in vitro during static and cyclic loading. Scand J Dent Res 1983; 91: 488-95.
22 . Jørgensen KD: Dentale amalgamer. København: Odontologisk Boghandels Forlag, 1976.
23 . Zander HA: Effect of silicate cement and amalgam on the gingiva. J Am Dent Assoc 1957; 55: 11-5.
24 . Snaedal J, Johannesson T, Jonsson JE, Gylfadottir G: The effects of nicotine in dermal plaster on cognitive functions in patients with Alzheimer's disease. Dementia 1996; 7: 47-52.
25 . App GR: Effect of silicate, amalgam, and cast gold on the gingiva. J Prosthet Dent 1961; 11: 522-32.
20
26 . Trott JR, Sherkat A: Effect of class II amalgam restorations on health of the gingiva: a clinical survey. J Can Dent Assoc 1964; 30: 766-70.
27 . Sotres LS, Van Huysen G, Gilmore HW: A histologic study of gingival tissue response to amalgam, silicate and resin restorations. J Periodontol 1969; 40: 543-6.
28 . Turgeon J, LeMay LP, Cleroux R: Periodontal effects of restoring proximal tooth surfaces with amalgam: a clinical evaluation in children. J Can Dent Assoc 1972; 38: 255-6.
29 . Lichtenberg H: Symptoms before and after proper amalgam removal in relation to serum globulin reaction to metals. J Orthomol Med 1996; 11: 195-204.
30 . Trivedi SC, Talim ST: The response of human gingiva to restorative materials. J Prosthet Dent 1973; 29: 73-80.
31 . Bartold P, Wiebkin O, Thornard J: The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontal Res 1984; 19: 390-400.
32 . Pizzorno JE, Murray MT. Textbook of Natural Medicine. Churchill Livingstone,1999; 2: 1487-9.
33 . Cohen BI, Penugonda B: Use of inductively coupled plasma-emission spec-troscopy and mercury vapor analyses to evaluate elemental release from a high-copper dental amalgam: a pilot study. J Prosthet Dent 2001; 85: 409-12.
34 . Frostell G: Kost och karies. In: Ericsson Y, red. Kariologiska principer: nordisk lärobok i kariologi. Stockholm: Tandläkarförlaget, 1980: 187-215.